Search Thermo Fisher Scientific
Coelenterazine & Synthetic Analogues
- Coelenterazine and coelenterazine derivatives complex with aequorin to offer luminescent Ca2+ readout in cells
- Luminescent readout eliminates common cellular fluorescence interference issues
- Highly sensitive, allowing measurement of Ca2+ concentrations from 0.1 µM to 100 µM levels
- Invitrogen offers a portfolio of 6 Coelenterazine proteins including 5 derivatives offering differing Ca2+ affinities and spectral emissions
- Native Coelenterazine as well as all 5 analogs are available together in a Sampler Panel for comparison and evaluation
Coelenterazine & coelenterazine derivatives complex with aequorin to offer Ca2+ readout in cells
Aequorin is a calcium-sensitive bioluminescent protein from the jellyfish Aequorea victoria that has been used extensively as a microinjectable calcium indicator in cells. The aequorin complex comprises a 22,000-dalton apoaequorin protein, molecular oxygen and the luminophore coelenterazine (Figure 1). When three Ca2+ ions bind to this complex, coelenterazine is oxidized to coelenteramide, with a concomitant release of carbon dioxide and blue light (Figures 2 and 3). The approximately third-power dependence of aequorin's bioluminescence on Ca2+ concentration gives it a broad detection range, allowing the measurement of Ca2+ concentrations from ~0.1 µM to >100 µM.
In addition to native coelenterazine (C-2944), we offer five derivatives of coelenterazine designated cp, f, h, hcp and n by Shimomura and colleagues that confer different Ca2+ affinities and spectral properties to the aequorin complex (Table 1). Like native coelenterazine, these five derivatives can be used to reconstitute the aequorin complex both in vivo and in vitro. Aequorin reconstituted with coelenterazine hcp shows very favorable characteristics, including a fast response to binding Ca2+ and the highest luminescence quantum yield of these five coelenterazine derivatives. Aequorins containing the cp, f or h form of coelenterazine are reported to exhibit relative intensities that are 10–20 times that of apoaequorin reconstituted with native coelenterazine. Knight and co-workers reported that aequorin reconstituted with coelenterazine h is more sensitive to Ca2+ than is the native complex, thus providing a valuable tool for measuring small changes in Ca2+ concentrations. Coelenterazine n is reportedly the most useful low-sensitivity coelenterazine, producing an apoaequorin/coelenterazine complex that exhibits 10,000-fold lower luminescent intensity than the apoaequorin/coelenterazine hcp complex.
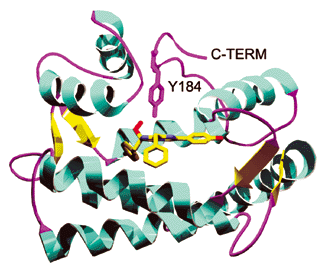
Figure 1. Ribbon representation of the aequorin/coelenterazine complex showing the secondary structural elements in the protein. Coelenterazine and the side chain of Tyr 184 are shown as stick representations. Reproduced with permission from.

Figure 2: The Ca2+-induced luminescence emission spectrum of native aequorin incorporating the coelenterazine luminophore (C2944).

Figure 3: Ca2+-dependent generation of luminescence by the aequorin complex, which contains apoaequorin (APO) and coelenterazine (C2944).
Table 1.
| Cat # | MW | Storage | Soluble | Abs | EC | Em | Solvent | Notes |
|---|---|---|---|---|---|---|---|---|
| C2944 | 423.47 | FF,D,LL,AA | MeOH | 429 | 7500 | see Notes | pH 7 | 1, 2, 3 |
| C6780 | 407.47 | FF,D,LL,AA | MeOH | 437 | 9500 | see Notes | MeOH | 1, 2 |
- Coelenterazine complexes with apoaequorin emit calcium-dependent bioluminescence. Bioluminescence emission maxima (relative intensity at 100 nM Ca2+) are as follows: C2944, 466 nm (1); and C6780, 466 nm (16).
- Do NOT dissolve in DMSO.
- Aqueous solutions of coelenterazine (>1 mM) can be prepared in pH 7 buffer containing 50 mM 2-hydroxypropyl-β-cyclodextrin.
Ordering Information
For Research Use Only. Not for use in diagnostic procedures.