Search Thermo Fisher Scientific
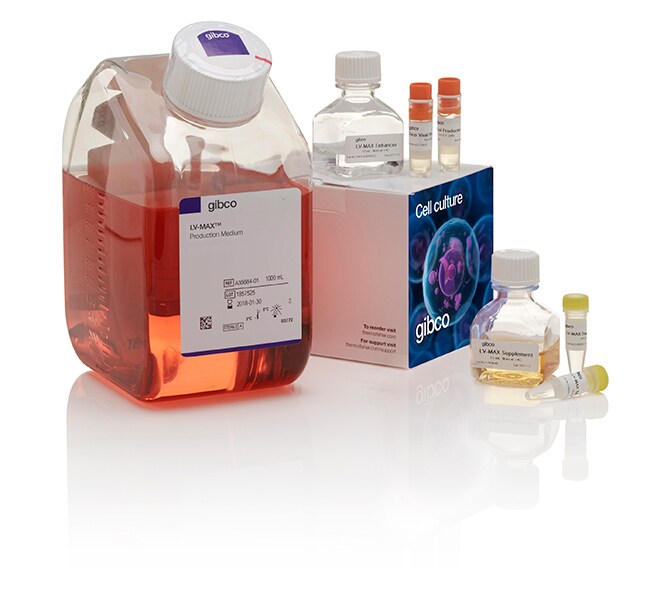
LV-MAX™ Lentiviral Production System
| Catalog Number | Quantity |
|---|---|
| A35684 | 1 Kit |
The LV-MAX Lentiviral Production System provides:
• High titer—greater than 1 x 108 TU/mL (unconcentrated LVVs-GFP)
• Scalable suspension system—from 96 deep-well plate to 2-L bioreactor
• Serum-free—eliminating animal-origin components and associated risks
• Easy, robust culture and transfection protocols—fits into current transient production workflows
The components included in the LV-MAX Lentiviral Production System are: two vials of frozen Viral Production Cells, one liter of LV-MAX Production Medium, and one LV-MAX Transfection Kit sufficient to produce 0.3 L of lentivirus. All components of the LV-MAX Lentiviral Production System are also available for purchase separately. Also required, but not included in this kit, is Gibco Opti-MEM I Reduced Serum Medium (Cat. No. 31985062).
• 0.3 L LV-MAX Transfection Kit. Store at 2–8°C, protect from light.
• 2 vials of Viral Production Cells. Store in liquid nitrogen until ready to use. Do not store the cells at -80°C.
Frequently asked questions (FAQs)
Customers who viewed this item also viewed
Documents & Downloads
Certificates
Safety Data Sheets
Scientific Resources
Product Information
Limited Use Label Licenses (LULL)
Notice to Purchaser: The purchase of this product conveys to the purchaser the limited, non-transferable right to use the purchased amount of the product and progeny, to perform internal research and development for the sole benefit of the purchaser. The purchase of this product does not grant the purchaser any additional rights, including, without limitation: (a) the right to use the product or its components in commercial applications of any kind, including bioproduction (e.g. use of the product to manufacture therapeutic agents or diagnostic test components, such as proteins, antibodies, viral particles or virus-like particles), quality control and commercial services such as reporting the results of purchaser's activities for a fee or other form of consideration; (b) the right to use the product or its components as a therapeutic agent or diagnostic test component; (c) the right to use the product or its components to produce material for use in human clinical trials; or (d) the right to transfer or resell the product or its components in any form, progeny or derivative. For information on obtaining additional rights, please contact outlicensing@thermofisher.com, Licensing and Commercial Supply, Thermo Fisher Scientific, 5823 Newton Drive, Carlsbad, CA, 92008, United States.
'